Feline Asthma
Do Cats Get High Blood Pressure?
How to Survive the Cat with Chronic Sneezing
Constipation in Cats
by Susan Little DVM, Dipl ABVP (Feline)
Constipation is the infrequent and difficult evacuation of feces with retention of feces within the colon and rectum. Obstipation is intractable constipation. The typical feline patient is middle-aged and male. Many cats have one or two episodes of constipation without any further problems. However, chronic
constipation and obstipation may result in megacolon where a dilated large bowel is poorly responsive to therapy. Cats with idiopathic megacolon have generalized dysfunction of colonic smooth muscle. Some of the more common underlying causes of constipation include:
Drugs: especially opiates, anticholinergics, sucralfate.
Behavioral: Stressors, e.g., changes in the home or routine Litter box aversion.
Difficulty defecating: Pain in rectal or perineal area.
Orthopedic problems: arthritis.
Neurologic problems.
Fecal factors: Excessive fecal bulk
Colon factors: Mass: intra-or extra-luminal, Obstruction due to narrowed pelvic canal, e.g. from previous trauma
Miscellaneous: Dehydration, e.g. due to chronic renal disease .
Idiopathic megacolon In one review, 62% of cases were due to idiopathic megacolon and 23% to pelvic canal narrowing.S Whenever possible, the underlying cause should be identified and corrected.
The clinical signs of constipation are typically obvious to the owner, such as tenesmus, and scant hard dry feces, sometimes with blood. However, cats will also strain in the litter box due to lower urinary tract obstruction and owners may misinterpret this as due to constipation. Occasionally, constipated cats will have intermittent diarrhea as the colon is irritated due to hard dry fecal matter. Other clinical signs are non-specific, such as vomiting, inappetence and lethargy.
Physical examination confirms the presence of large amounts of feces in the colon sometimes accompanied by abdominal pain. The colon often palpates as a long firm tube or feces may be palpated as discrete concretions. A careful evaluation (e.g., musculoskeletal system, caudal spinal cord function, anorectal area) should be made for underlying causes. A rectal exam should be performed, under sedation if necessary, for masses, pelvic fracture malunion and anal gland abnormalities. A minimum database (CBC, serum chemistries/electrolytes, urinalysis) should be assessed, especially to determine hydration and electrolyte status and identify underlying diseases such as chronic renal disease. Survey abdominal radiographs are useful to confirm the diagnosis and assess severity as well as to evaluate for potential underlying causes, such as previous pelvic trauma and arthritis. The diameter of the colon on a lateral view should be approximately the same length as the body of the 2nd lumbar vertebra.10 Enlargement of the colon beyond 1.5 times the length of the body of the 5th or 7th lumbar vertebra has been proposed as indicating chronic dysfunction and megacolon.»-10 One study of 11 cats with megacolon found the mean diameter of the colon was 2.7 times greater than the length of the 7th lumbar vertebra (median: 2.4, range 1.8-3.3). In some cases, further diagnostics such as a barium enema or colonoscopy may be warranted.
The first step in management is correction of dehydration with intravenous fluid therapy followed by removal of obstructing feces. One or two doses of a 5 ml microenema containing sodium lauryl sulfoacetate (MicroLax) is easily administered and will usually produce results within 20-30 minutes in mildly affected cats. Obstipated cats will require warm water or isotonic saline enemas (5-10 ml/kg). Safe additions to the water include mineral oil (5-10 ml/cat), or docusate (5-10 mL/cat). Soaps or detergents may be irritating to an already compromised colonic mucosa. Lactulose solution can also be administered as an enema (5-10 ml/cat). Sodium phosphate containing enemas must not be used as they can induce life-threatening hypernatremia, hyperphosphatemia and hypocalcemia in cats. Enemas are administered slowly with a lubricated 10-12 French feeding tube. In severe cases, manual manipulation of the feces via abdominal palpation or per rectum (manual disimpaction) under general anesthesia with endotracheal intubation (in case of vomiting) is also required. In these cases, opioids should be administered for pain relief.
An alternative to enemas is administration of an oral polyethylene glycol (PEG 3350) solution (e.g., CoLyte, GoLytely). A nasoesophageal tube is placed and the solution is given as a slow trickle (6-10 ml/kg/hour) over 4-18 hours. Defecation usually results in 6-12 hours. In a retrospective study of 9 cats, median time to defecation was 8 hours and the median total dose of PEG 3350 was 80 ml/kg.?
No adverse effects were noted.
In addition to management of any underlying conditions, long term medical treatment involves a combination of prokinetic agents, laxatives and dietary therapy. Cisapride stimulates contraction of feline colonic smooth muscle.“ A typical starting dose is 2.5 mg/cat BID, PO and it is better absorbed when given with food. Doses up to 7.5 mg/cat, TID have been reported. The drug is only available from compounding pharmacies in most countries. It has been withdrawn from the human market due to the occurrence of life-threatening arrhythmias in predisposed individuals (not known to occur in cats). It may be prudent to advise clients handling cisapride to wear gloves. Hyperosmotic laxatives include lactulose and PEG 3350; they stimulate colonic fluid secretion and propulsive motility. The dose of lactulose solution is 0.5 mL/kg, PO, BID-TID. Lactulose is also available as crystal meant to be mixed in liquids for human use (Kristalose). A suggested dose is 3/4 tsp. BID with food. PEG 3350 is available as a powder meant to be mixed in liquids for human use (MiralAX). A suggested dose for cats is 1/8 to 1/4 tsp. BID in food.
Dietary therapy has included the use of high fiber diets (>20% on as fed basis) and low residue diets. Increased dietary fiber increases the production of short chain fatty acids which stimulate feline colonic smooth muscle contraction. Dietary fiber is also a bulk laxative and will increase fecal bulk, which will not be beneficial for all patients. Feeding a canned diet is often recommended to reduce fecal bulk and to ensure adequate water intake and hydration. Psyllium powder can be mixed with canned food at 1-4 tsp. SID-BID. A certain amount of trial and error is necessary to determine the best diet type for an individual patient.
Recently, a moderate fiber, psyllium-enriched dry extruded diet was introduced for management of gastrointestinal conditions in cats (Royal Canin Gastro Intestinal Fiber Response). In an uncontrolled study by the manufacturer, 66 cats with recurrent constipation were successfully treated with the Fiber Response diet. The diet was well tolerated and palatable. Most cats improved within 2 months and were either maintained on diet alone or with decreased doses of cisapride and lactulose than previously used.
It is also important to ensure adequate water intake by various methods, such as feeding canned diets. Most water bowls designed for cats are too small; cats dislike having their whiskers touch the side of containers. Dog water bowls are larger and more appropriate. Other methods for increasing water intake include:
Mix water with dry diets 1:1 Flavor water with frozen cubes of meat or fish broth Try distilled or filtered water, especially if the tap water supply is heavy in minerals or chlorine Ensure water is fresh every day, and provide multiple water bowls Ensure the water bowls are kept clean
Keep food and water bowls away from the litter box – Feed multiple smaller meals instead of one or two larger meals – Provide a moving source of water such as a pet water fountain
Litter box modification may be helpful for cats with arthritis. Most cat litter boxes are too small and have high sides. A winter boot tray or an under-the-bed type of storage box with low sides is a better alternative to make access easier. The litter box should also be in an accessible but private area, avoiding the need to navigate stairs if possible.
Subtotal colectomy (95-98% excision, with preservation of the ileocolic junction) should be considered for cats refractory to medical and dietary therapy. Long term outcome is considered excellent. Most patients will experience transient diarrhea in the immediate post-operative period (1-6 weeks). In a small number of patients, diarrhea will persist.
- 57:580, 1996.
Early Age Altering of Kittens
by Susan Little DVM, Dipl ABVP (Feline)
Refers to spay/neuter before the traditional age of six to eight months of age
- Desirable approach to pet overpopulation control for shelters; enables pre-adoption altering
- Pedigreed cat breeders may request early age altering for pet kittens
- Major North American veterinary organizations have position statements supporting the concept
Perceived detrimental effects include:
- Stunted growth
- Obesity
- Behavioural changes
- Increased risk of disease, including lower urinary tract disease
Growth: altering at any age prior to seven months may delay physeal closure and result in increased long bone length
- University of FL (Stubbs et al, 1996): evaluated skeletal growth in cats altered at seven weeks, seven months and those left intact; delayed closure of the growth plate of the radius was seen in cats altered at both seven weeks and seven months of age
- University of MN (Root el al, 1997): radius length was longer in cats altered at both seven weeks and seven months versus intact cats
Obesity: a multifactorial problem involving diet, exercise, age and other factors; Root et al (1996) showed that altered cats require fewer calories than intact cats regardless of the age at which they were neutered; male cats altered at both seven weeks and seven months required 28% less calories than intact male cats; female cats altered at both seven weeks and seven months required 33% fewer calories than intact female cats
Behaviour:
- University of FL study (Stubbs et al, 1996): behaviours were similar between both groups of altered cats; sexually intact cats demonstrated less affection toward humans and more interspecies aggression
- Cornell University study, 1660 cats with median follow up time of 4 years (Spain et al, 2004): early altered cats showed more shyness and less hyperactivity than cats neutered after six months of age; early altered male cats showed less aggression to veterinarians, less urine spraying but more occurrences of hiding
- Mercer University study (Wright, 2001): 127 kittens, split litter design, followed for one year, no differences in behaviours based on age at altering
- Texas A&M Study (Howe et al, 2000): 263 cats (188 altered at under six months of age), followed for three years, no difference in behaviour problems
Disease risk:
- University of FL, University of MN studies: urethral diameter is not affected by age at neutering in male cats; feline lower urinary tract disease is caused by a wide variety of factors, including diet
- Cornell University study (Spain et al, 2004): concluded neutering before 5.5 months of age is not associated with any serious long-term outcomes, showed important benefits such as less asthma, gingivitis and bite wound abscesses
- Texas A&M study (Howe et al, 2000): 263 cats (188 altered at under six months of age), followed for three years, no increased disease risk as compared to traditional age surgeries
Anesthesia and Surgery:
- Pediatric animals distribute and metabolize drugs differently, must be careful with drug selection and doses
- Surgical benefits to early age altering:
- Less bleeding
- Improved visualization
- Small size of organs means shorter surgery time
- More rapid recoveries, less patient discomfort
- Very low complication rate
- Anesthetic complication rates are low:
- Faggella and Aronsohn’s study of 96 kittens reported no complications. 2. Texas A&M University (Howe et al, 1997): 779 shelter cats altered by 4th year vet students, early age altering did not increase morbidity or mortality during the intra- or postoperative period (seven days after surgery). Kittens altered at less than 12 weeks of age actually had a lower postoperative complication rate (6.5%) than those altered at greater than 23 weeks of age (10.8%).
Five Rules for Successful Early Age Altering:
- Kittens should have complete physical exam; have their first vaccination and treatment for parasites; postpone surgery if any illness or abnormality found (including cryptorchidism)
- Weigh each kitten to nearest 100 grams, calculate drug doses carefully 3. Combat hypoglycemia: withhold food for only three to four hours; feed a small meal within one hour of recovery; administer 50% dextrose orally to kittens with prolonged recoveries or those that will not eat post-op
- Decrease stress: keep litters together before surgery in warm, quiet environment; minimize handling; avoid IV injections; reunite litters as soon as possible after recovery
- Combat hypothermia: insulate against cold surfaces, minimize hair coat clipping, avoid alcohol in preps, warm prep solutions, monitor rectal temperature, use supplemental heat sources (warm blankets, hot water bottles, heat lamps, etc)
us, OH, 2001, pp. 89-90.
Feline Blood Types and Neonatal Isoerythrolysis
by Susan Little DVM, Dipl ABVP (Feline)
Cats have one blood group system with three blood types: type A, type B, and type AB.
Based on the fact that each individual has two sets of chromosomes, these are due to different forms (alleles) of the same gene.
Thus, only cats carrying two copies of the B allele (genotype homozygous B/B) will have blood type B.
Cats with blood type A may have two copies of the A allele (homozygous AA) or one only copy (heterozygous A/B).
Type A is completely dominant over type B. The third blood type, type AB, appears to be a third form of the same gene, but it is rare.
Type A is the most common feline blood type, present in up to 94%-99% of all domestic shorthair and longhair cats in the United States.
The frequency of the feline blood groups varies both by breed and by location within the United States. The lowest frequency of type B cats is in the Northeast and North Central/Rocky Mountain regions. Higher frequencies of type B cats are found on the West Coast, peaking in the Northwest with 6% type B cats.
Siamese cats and related breeds with oriental blood have thus far all been shown to have type A blood. The American Shorthair breed, due to its close relationship to non-pedigreed shorthair cats, is also largely blood type A. However, some other breeds may have astoundingly high numbers of type B cats. The frequency of the blood types does not vary geographically for pedigreed cats.
Frequency of Blood Types in Pedigreed Cats
(From surveys conducted by the University of Pennsylvania over past 15 years)
| Breed | % Type A | % Type B |
| Abyssinian | 86 | 14 |
| American Shorthair | 100 | 0 |
| Birman * | 82 | 18 |
| British Shorthair * | 64 | 36 |
| Burmese | 100 | 0 |
| Cornish Rex | 67 | 33 |
| Devon Rex | 59 | 41 |
| Exotic Shorthair | 73 | 27 |
| Himalayan | 94 | 6 |
| Japanese Bobtail | 84 | 16 |
| Maine Coon | 97 | 3 |
| Norwegian Forest Cat | 93 | 7 |
| Oriental Shorthair | 100 | 0 |
| Persian | 86 | 14 |
| Russian Blue | 100 | 0 |
| Scottish Fold * | 81 | 19 |
| Siamese | 100 | 0 |
| Somali * | 82 | 18 |
| Sphynx * | 83 | 17 |
| Tonkinese | 100 | 0 |
(* indicates breeds with some type AB cats)
All blood type B cats have strong antibodies against type A blood cells as of three months of age. Blood type A cats generally have very low anti-B antibody titers. It is very important to note that these antibodies are naturally occurring; unlike other species, no previous pregnancy or transfusion is necessary for antibody development. The strong anti-A antibodies in type B cats are important in two situations: blood transfusion reactions following the administration of type A blood to type B cats and neonatal isoerythrolysis (NI or hemolysis of the newborn) due to the newborns’ type A or AB erythrocytes being attacked by the anti-A antibodies in the type B queen’s colostrum. The anti-B antibodies have not been shown to cause NI, but can lead to transfusion reactions if type B blood is given to type A cats. Fortunately these situations can now be avoided.
Neonatal isoerythrolysis is an immunologic, genetic problem seen in cats, but not dogs. It may be responsible for a large proportion of fading kittens and neonatal deaths in some pedigreed catteries, where the blood type of breeding cats is unknown. NI occurs in blood type A kittens born to a type B queen mated to a type A male. If the tomcat is homozygous (A/A), then all the kittens in the litter will be blood type A and at risk for NI. If the male cat is heterozygous (A/B), then 50% of the offspring would be expected to be heterozygotes with blood type A (genotype A/B) and at risk for NI. This problem can also occur in type AB kittens born to type B queens.
When kittens nurse from the queen after birth, they receive colostrum that contains antibodies to protect them against common viral infectious diseases, but also antibodies against blood types. The kitten’s digestive tract is able to absorb these antibodies, which pass into their bloodstream,
for about the first 12-24 hours of life. After that time, “gut closure” occurs in the neonate that prevents absorption of any antibodies. When type A or AB kittens nurse on a type B queen during the first day of life, they receive anti-A antibodies in the colostrum, which in turn get into the blood stream and bind to their red blood cells and destroy them (this is known as isoerythrolysis).
Clinical signs of NI are variable. Large variations in clinical signs may be due to ingestion of varying degrees of anti-A antibodies in colostrum or as yet undetermined factors. Typically, kittens are born healthy and nurse well. Clinical signs may appear rapidly, with some kittens dying suddenly, within hours. Other kittens will stop nursing within the first three days of life with suggestive signs of failure to thrive, red-brown urine, jaundice, and anemia. As they deteriorate, lethargy, weakness, rapid breathing, a very slow or rapid heart rate, as well as collapse, and eventually death may occur. Some kittens appear to have subclinical disease with no obvious clinical signs. If tested, they may have anemia, however. Surviving kittens may develop damage to the skin of the tail tip up to two weeks later.
Kittens with signs of NI should immediately be removed from the queen to prevent further absorption of antibodies. They need be removed from the queen for only about the first 24 hours of life since no antibodies are absorbed after that time. The kittens should be foster-nursed by a queen with type A blood if available, or hand-fed a kitten milk replacer. Kittens with severe anemia require a transfusion, but unfortunately these efforts are rarely if ever successful.
Since the mortality rate with NI is high, the predisposing situation should be prevented by knowing the blood type of breeding cats in breeds with known occurrences of type B blood. In breeds with low type B blood frequencies and catteries with mostly type A breeders, cats that are blood type B may not be used for breeding, in order to minimize future problems with NI. Many breeders are now recording each cat’s blood type on pedigree charts to facilitate breeding decisions. Blood typing can be done in a referral laboratory or by using in-clinic blood typing ®
cards (RapidVet-H , DMS Laboratories, 2 Darts Mill Rd., Flemington, NJ 08822, 1-800-567- 4367). The rare type AB results may be due to autoagglutination or other technical difficulties and should therefore be confirmed in a reference laboratory by a tube assay such as the laboratory at the University of Pennsylvania uses.
When a breeding between a type B queen and a type A tom must be done, the breeder should endeavor to be present at the birth of the kittens to prevent nursing from the queen. The easiest method is to breed a second queen with type A blood a little earlier, so that the litters from the 2 queens can be exchanged. The second queen’s litter should be over 24 hours old in order to exchange litters. This has the added advantage of potentially allowing the kittens to acquire some passive immunity from the foster queen’s milk. Queen’s milk contains similar quantities of antibodies as in colostrum. The other option is to hand feed the kittens with a commercial milk replacer. Experience suggests that these kittens will do fine, although they will not have any protective antibodies. In either case, the kittens can be returned to their own mother in 24 hours. Some breeders who elect to hand feed milk replacer allow the kittens to stay with the mother for care, but fit the queen with a type of body stocking so that the kittens are unable to nurse. However there are some clever neonates that still find their way to the nipples and suffer the consequences.
Feline Pediatrics: How to Treat the Small and the Sick
Susan Little, DVM, DABVP (Feline Practice)
Bytown Cat Hospital Ottawa, Ontario, Canada
Feline Pediatrics: How to Treat the Small and the Sick*
Most veterinarians have been presented with kittens that have failed to thrive. These patients are challenging due to their small size, their unfamiliar physiology, and the tendency for their status to deteriorate quickly. The most common general causes of illness and failure to thrive are maternal, gesta-
tional, environmental, genetic, and infectious factors.1,2 In much of the veterinary literature, the neonatal period is defined as the first 4 weeks of life. However, it is clinically useful to consider defined risk periods: the first 4 days of life (when many problems are related to labor and delivery or the environment); between 21 and 28 days of age (when important changes leading to neurologic and behavioral maturation take place); and weaning (4 to 6 weeks of age).
Examination of Neonatal Kittens
There are many clinically relevant physiologic differences between neonatal kittens and adult cats3 (TABLE 1), and very young kittens cannot be approached as small adults. Sick neonates should be examined as soon as possible, using a systematic approach that includes a complete history of the kitten, litter, and queen; examination of the kitten and queen; and diagnostic tests.4,5 Kittens younger than 4 weeks should be examined with the queen present when possible (unless prohibited by the queen’s temperament).Start with a complete medical history for the kitten in question as well as for littermates. It may also be helpful to have a medical history for the queen, if available (e.g., illness, nutrition, vaccina-
tions), and information about the labor and delivery, especially for kittens younger than 2 weeks. If it is not the queen’s first litter, information should also be gathered on previous litters and any previous problems with labor and delivery. Investigate the kitten’s home environment, noting temperature and humidity, sanitation, ventilation, population density, the presence of other pets and small children, and prevalence of infectious diseases and parasites.
Neonates should be handled gently, on a clean, warm surface. Wash your hands and wear gloves. Simple equipment will suffice for neonatal examinations: a gram scale, pediatric rectal ther-
mometer, otoscope with infant cones, penlight, and stethoscope with an infant bell and diaphragm.
Before handling the kitten, observe its body condition and response to the environment, including alertness, posture, loco-motion, and respiratory rate and character. Healthy neonates have a strong suckle reflex that is, by comparison, normally less strong than that of a healthy puppy. Normal body temperature for neonatal kittens is 97°F to 98°F (36°C to 37°C). The rectal temperature rises slowly, reaching 100°F (38°C) by about 4 weeks of age. For the first 2 weeks of life, kittens are essentially poikilothermic and lack a shiver reflex. They gradually become homeothermic after 14 days of age, but are still susceptible to environmental conditions and may become hypothermic easily.
If the birth date is unknown, attempt to establish an estimated age for the kitten by using body weight and inspection of the dentition. The typical kitten birth weight is 90 to 110 g (range: 80 to 140 g), although there is considerable variation by and within breed.6 Normal kittens gain 50 to 100 g/wk (10 to 15 g/d) and should double their birth weight before 2 weeks of age. Low birth
weight is a common cause of mortality, with kittens weighing under 75 g at birth at highest risk. The first deciduous teeth to appear are the incisors and canines at 3 to 4 weeks of age. The premolars erupt at about 5 to 6 weeks of age. The dental formula for deciduous teeth is 2(I3/3, C1/1, P3/2) = 26; there are no deciduous molars.
Developmental milestones may also be helpful in estimating age (TABLE 2), although delayed development may occur in kittens with low birth weight and poor weight gain. Ex-amples include uncoordi-nated walking by 21 days
and coordinated walking by 28 days.Inspect the neonate for
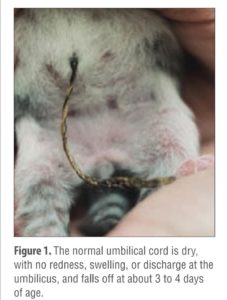
gross anatomic abnormali-ties, such as cleft palate or lip, umbilical hernia or
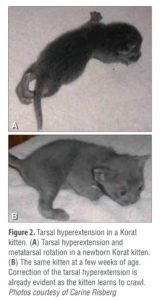
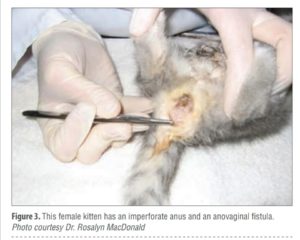
infection (FIGURE 1), open fontanelle, limb deformi-ties (FIGURE 2), chest wall deformities, and nonpatent urogenital or rectal openings
(FIGURE 3). Kittens younger than about 3 weeks cannot eliminate urine and feces voluntarily. Evaluate a kit-ten’s micturition and defe-cation reflexes using a cotton ball moistened with mineral oil or warm water to stim-
ulate the anogenital area.
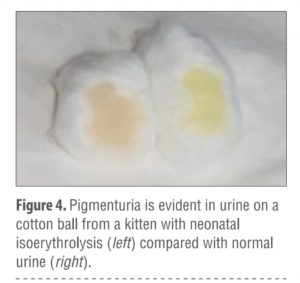
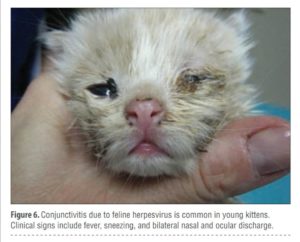
 Diarrhea is present in about 60% of sick neonatal kittens and may cause sig-nificant fluid loss. Hematu-ria or pigmenturia may be signs of urinary tract infection, trauma, or neonatal isoerythrolysis (FIGURE 4). Neonatal isoerythrolysis may be a common problem in some breeds (e.g., British shorthair, Cornish rex, Devon rex) with a high percentage of individuals with blood type B. BOX 1 lists selected common con-genital defects in neonatal kittens that are apparent around the time of birth.
Diarrhea is present in about 60% of sick neonatal kittens and may cause sig-nificant fluid loss. Hematu-ria or pigmenturia may be signs of urinary tract infection, trauma, or neonatal isoerythrolysis (FIGURE 4). Neonatal isoerythrolysis may be a common problem in some breeds (e.g., British shorthair, Cornish rex, Devon rex) with a high percentage of individuals with blood type B. BOX 1 lists selected common con-genital defects in neonatal kittens that are apparent around the time of birth.
The eyes should be inspected for abnormalities of the globe or eyelids and for neonatal ophthalmia (before the eyes open) or conjunctivitis (after the eyes open). A menace reflex and pupillary light responses do not appear until 28 days of age or later. A divergent strabismus may be present and is normal until about 8 weeks of age unless hydrocephalus is present. Evaluation of the fundus is difficult until about 6 weeks of age.
The pinnae should be inspected for evidence of trauma, parasites such as ear mites, and skin disease.The ear canals are not easy
How to Treat the Small and the Sick.
Inspect with an otoscope un- til after 4 weeks of age. The neonate’s haircoat should be clean and shiny. Healthy neonatal kittens may have somewhat hyperemic mucous membranes until 7 days of age (although hyperemia may also be a sign of dehydration),whereas sick neo-nates often have pale, gray, or cyanotic mucous membranes. Kittens with cyanotic mucous membranes have a poor prognosis.
The cardiovascular system undergoes dramatic changes as the heart takes over the functions previously performed by the feto-maternal circulation. One important physiologic difference between neonates and adults is the higher neonatal heart rate. The normal neonatal heart rate can be >200 bpm (range: 220 to 260 bpm).
The normal respiratory rate is 15 to 35 breaths/min. Functional murmurs may be present in neonates due to anemia, hypopro-teinemia, fever, or sepsis. Innocent murmurs not associated with disease are more common in puppies than kittens; murmurs present after 4 months of age should be investigated. Congenital heart disease may be associated with murmurs that are loud and
accompanied by a precordial thrill. The most common congenital heart diseases in kittens are tricuspid valve dysplasia and ventricular septal defect.
Abdominal palpation can be performed with care; in the first few days of life, abdominal pressure during palpation may induce regurgitation of stomach contents and aspiration. A full abdomen is normal in a well-fed kitten, but an enlarged abdomen in an ill kitten may indicate aerophagia. The normal liver and spleen may not be palpable; the kidneys are frequently palpable. The stomach may be palpable if it is full. The intestinal tract is palpable as fluid-
filled bowel loops that should be freely movable and nonpainful.
The normal urinary bladder is also palpable, movable, and non-painful.
Diagnostics
For venipuncture, position the kitten in dorsal recumbency with the forelegs drawn back toward the abdomen and the head and neck extended. Draw blood from the jugular vein using a 1-mL syringe with a 25- or 26-gauge needle. Slow aspiration of blood is essential to avoid collapsing the vein. A small volume (0.5 mL) of blood can be used for the most critical tests (BOX 2).Blood chemistry and hematology values for neonates differ from those for adults (TABLE 3 and TABLE 4); most values normalize to adult levels by 4 months of age.8–10 Urine is collected for chemistry, sediment, and specific gravity analysis by stimulating the perineum; cystocentesis should be performed with great care in the very young because the bladder wall is easily damaged and because umbilical vasculature may still be patent and can be traumatized.
Urine specific gravity is 1.020 or lower in the first few weeks of life; adult values are reached by about 8 weeks of age.11 A fecal sample should be examined for common intestinal parasites such as Giardia and Isospora spp and roundworms using zinc sulfate centrifugation, a direct saline smear, and a Giardia fecal antigen test. Kittens as young as 2 weeks may be treated with pyrantel pamoate (5 to 10 mg/kg PO every 2 weeks).
Flea infestations should be treated aggressively, as they can cause life-threatening anemia. Young kittens can be bathed in pet-safe shampoo followed by thorough drying and combing of the hair-coat. Water-based pyrethrin sprays labeled for use in kittens may also be used. Nitenpyram is labeled for use in kittens at least 4 weeks of age and weighing at least 2 lb (0.9 kg). Most other flea control products are labeled for use in kittens from 8 weeks.
How to Treat the Small and the Sick age, although, anecdotally, selamectin has been used in kittens as young as 6 weeks of age. Ear mite (Otodectes) infestations are best treated with topical ivermectin. One product (Acarexx, Boehringer Ingelheim) has been demonstrated as safe in kittens as young as 4 weeks of age.

Testing for FeLV and FIV is an important part of both wellness care and investigation of illness (TABLE 5). Recommendations for FeLV and FIV testing in kittens have been recently published and should be reviewed.
Necropsy is underutilized as a diagnostic tool for multicat environments such as shelters or catteries. Necropsy results may provide information necessary to save remaining littermates or a future litter. For the best results, the whole body should be submitted (refrigerated, not frozen) to a qualified pathologist. If necessary, freezing is preferable to autolysis.
Basic Therapeutics
Rapid identification of illness and prompt intervention are the keys to success when treating ill kittens. Often the exact cause of a kitten’s illness is not apparent at the time of presentation, and therapy must be focused on supportive care. Initial therapy may include supplemental warmth, hydration, glucose administration, and nutritional support. Awareness of physiologic differences between neonatal and adult cats is important, and information about these differences should be reviewed.
Severe hypothermia occurs when the kitten’s rectal temperature is <94°F (34.4°C) and is associated with depressed respiration, impaired function of the immune system, bradycardia, and ileus. Hypothermic kittens should be rewarmed slowly, over 2 to 3 hours or more, to a maximum rectal temperature that is age appropriate.
Warming too rapidly may cause increased metabolic demand, resulting in dehydration, hypoxia, and loss of cardiovascular integrity. An incubator or oxygen cage is a good way to accomplish rewarming, but hot water bottles and heating lamps can also be used with very careful monitoring. For severely hypothermic kittens, fluids warmed to 95°F to 98°F (35°C to 37°C) may be administered via the intravenous (IV) or intraosseous (IO) route (depending on age).
Never attempt to feed a hypothermic kitten, as aspiration pneumonia due to gastrointestinal hypomotility and regurgitation is a significant risk. Monitor closely for recurrence of hypothermia after rewarming.Clinical hypoglycemia occurs when the blood glucose level is <3 mmol/L (50 mg/dL) and is a common problem for sick neonates due to kittens’ immature liver function and rapid depletion of glycogen stores. Hypoglycemia may be caused by vomiting, diarrhea, sepsis, hypothermia, or inadequate nutritional intake.
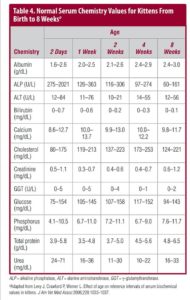
Kittens with hypoglycemia will be weak and lethargic and may be anorectic. If the kitten is not hypothermic or dehydrated, periodically administer 5% to 10% dextrose orally by gastric tube at 0.25 to 0.50 mL/100 g body weight until the kitten is stronger and normoglycemic (FIGURE 5).
Then begin feedings of kitten milk replacer if a lactating foster queen is not available. Critically ill neonates may require a bolus infusion of 12.5% dextrose IV or IO (0.1 to 0.2 mL/100 g or more), followed by a constant-rate infusion of 1.25% to 5% dextrose in a balanced electrolyte solution to prevent rebound hypoglycemia. Hypertonic dextrose solutions should not be administered sub-cutaneously because tissue sloughing may occur.
Dehydration occurs easily in neonatal kittens with hypoxia, hypothermia, diarrhea, vomiting, or reduced fluid intake. Neonates have poor compensatory mechanisms and immature kidney function. Daily urine output in kittens 1 month of age is 25 mL/kg compared with 10 to 20 mL/kg in adult cats.
Neonates also have higher fluid requirements than adults for reasons such as higher total body water (about 80% of body weight, compared with 60% in adults), greater ratio of surface area to body weight, higher metabolic rate, and less body fat. Hydration status may be difficult to assess in the youngest patients.
Skin turgor is not always a reliable test of hydration for kittens younger than 6 weeks because their skin contains less fat and more water than adult skin. The kitten’s mucous membranes should be moist and either slightly hyperemic or pink. Pale mucous membranes and a decreased capillary refill time indicate at least 10% dehydration. Neonatal urine is normally colorless and clear; in dehydrated kittens, the urine may be darker with a specific gravity >1.020.
If the kitten is normothermic and not in shock or cardiovascular collapse, warmed subcutaneous (SC) fluids can be administered, although absorption is slow in young kittens. If there is no gastrointestinal dysfunction, warmed oral fluids may be given.
These approaches are especially useful in the youngest and small-est patients (younger than 4 weeks).
If the kitten is moderately to severely dehydrated and large enough to facilitate IV therapy, IV fluid administration is the most effective. A mini-set (60 drops/mL) is used with a fluid or syringe pump or a burette. The cephalic or jugular vein can be catheterized with a 24-gauge, ¾-inch or 22- gauge, 1-inch catheter. Lactated Ringer solution is ideal for rehy-dration because lactate can be used as an energy source and 1.25% to 5% dextrose can be added if necessary.
Warmed fluids may be given as a slow IV bolus of 1 mL/30 g body weight (30 to 45 mL/kg), followed by a maintenance infusion of 80 to 120 mL/kg/d (8 to 12 mL/100 g/d) plus any ongoing losses.11,16 It is important to monitor fluid therapy closely; it is easy to overhydrate young kittens due to their immature renal tubular function. Hydration status can be monitored by several methods, but weighing the kitten every 6 to 8 hours on an accurate gram scale is useful and easily accomplished. Serial packed cell volume/total protein measurements may also be used. Electrolyte and glucose status should be monitored.
If it is difficult to achieve IV access, an alternative route for administration of fluids must be employed. The intraperitoneal route should be used cautiously in neonatal kittens due to the risk of inducing peritonitis or damaging blood vessels. IO access using the trochanteric fossa of the proximal femur is the best alternative to IV access in larger kittens; blood, fluids, and medications can be administered in this way, particularly in kittens about 4 weeks of age and older.Use a 20- to 22-gauge, 1-inch spinal needle or 18- to 25-gauge hypodermic needle as a catheter. Flow rates of up to 11 mL/min can be achieved by gravity. Use of cold fluids, too large a volume in a short time, or hypertonic or alkaline solutions will cause pain. IV access should be established as soon as possible.
Complications of IO administration include infection, extravasation of fluids, and bone and soft tissue trauma. Practical considerations, such as tolerance of stress, may dictate the use of SC fluid administration, at least initially. Careful monitoring of fluid absorption is required when the SC route is used.
Blood transfusions may be necessary in some sick neonatal kittens, particularly those with anemia due to fleas or intestinal parasites. Indications for blood transfusions are weakness, tachy-cardia, pale mucous membranes, and a hematocrit <15%. Blood from a compatible typed donor is diluted 9:1 with a citrate anticoagulant and given using a millipore blood filter via the IV or IO route at a rate of 20 mL/kg over a minimum of 2 hours.
Immunity
Kittens receive almost all their passive immunity during the first 18 hours of life (before gut closure) with the ingestion of colostrum; there is little or no transplacental transfer of immunoglobulins in cats.17 The serum IgG nadir is reached at about 4 weeks of age due to catabolism of maternal IgG and correlates with a period of vulnerability to infection ( FIGURE 6). IgG levels then steadily increase as the kitten’s own adaptive immunity develops.
Failure of passive transfer can occur in kittens that have not ingested colostrum during the first critical hours. Correction of failure of passive transfer can be accomplished by SC injection of serum from an adult cat with a compatible blood type that has been screened for infectious diseases. The only study in the literature used 15 mL/100 g body weight, divided into three doses over 24 hours.18 The minimum amount necessary is unknown.
Kittens with uncorrected failure of passive transfer start to produce IgG at about 4 weeks of age; they are therefore most vulnerable to infection from birth to at least 4 weeks of age.
Pyometra and CEH - by Susan Little DVM.Written by Lorraine Shelton
Cystic Endometrial Hyperplasia/Pyometra Susan Little DVM Diplomate ABVP (Feline) Bytown Cat Hospital Ottawa, Canada
a) Etiology:
It has typically been believed thatbecause cats are induced ovulators, the incidence of cystic endometrial hyperplasia(CEH)/pyometra is lower than in dogs.
However, recent studies have shown a great many catsare also spontaneous ovulators, and therefore may experience prolonged diestral periodswithout pregnancy.
Repeated pseudopregnancies may predispose the uterus to CEH, which isa disorder of proliferative and degenerative changes in the endometrium associated with aging.Most queens with pyometra were in estrus some time in the preceding 60 days.
Potter et al(1991) reported that 40% (16/40) of queens with pyometra or endometritis had CLs. Lawler et al(1991) reported that 67% (20/30) queens with pyometra had luteal phase ovaries.
Progesterone causes hyperplasia of the endometrium and endometrial glands.
Other effects ofprogesterone include inhibition of local leukocyte responses to infection in the uterus anddecreased myometrial contractility.
Estradiol causes an increase in the number of estrogen andprogesterone receptors in the endometrium. It also causes cervical dilation during estrus andtherefore allows bacteria that are part of the normal flora of the vagina (especially E. coli andStreptococcus spp) to ascend into the uterus.
It is normal for cats to have both aerobic andanaerobic bacteria in the vagina.
Younger cats have more vaginal bacteria than older cats, andcats in heat or pregnant have more bacteria than anestrus cats.
Vaginal cultures are thereforehard to interpret since the queen has normal bacterial flora.
This combination of ascendingbacteria and an abnormal endometrium predispose queens to pyometra.
b) Clinical symptomsand diagnosis:
- Cats with CEH may or may not have endometritis.
- CEH tends to be a chronicsubclinical condition and may be hard to diagnose definitively without biopsy of the uterus.
- Apresumptive diagnosis of endometritis may be made from response to antibiotic treatment,which should last for 2-4 months.
- Uterine pathology, mostly secondary to CEH andendometritis, is common in queens over 5 years of age.
- CEH is very common in unbred queensover 3 years of age. Perez et al (1999) found that 88.2% of queens older than 5 years in abreeding colony had CEH, versus a 30% incidence in queens 2-4 years old.
- A group of feralqueens also sampled had no CEH.
- They concluded that colony queens showed a predispositionto CEH that was correlated with elevated serum estradiol concentrations.
- CEH is one of themost important causes of infertility in catteries.
- Cats with advanced CEH are occasionallyfound with mucometra or hydrometra, characterized by variable amounts of mucus in theuterus.
- In hydrometra, the mucin is thin and watery.
- In mucometra, the mucin is thick or evensemisolid.
- Queens with either condition do not have bacterial infections and are not systemicallyill.
- The main symptom is abdominal distension, with or without a vaginal discharge.
- Theclinical signs of pyometra include a vulvar discharge, depression, dehydration, anorexia, fever,weight loss and a distended abdomen.
- Any abnormal vulvar discharge in an intact queen shouldbe assumed to be due to pyometra.
- However, 15-30% of queens have no vulvar discharge(closed cervix).
- Queens are often very meticulous in grooming, however, so evidence of thedischarge may be hard to find.
- A surprising number of queens with open cervix pyometras havelittle or no signs of systemic illness.
- Very occasionally, pyometra is found during a routineannual health examination.
- Polyuria and polydipsia are far less frequent in queens withpyometra than bitches.
- Most queens with pyometra will have a leukocytosis with a left shift.
- Thediagnosis can be affirmed by finding an enlarged uterus on radiographs or ultrasound.
- In somecases, the uterine enlargement is segmental, mimicking a pregnancy.
- Occasionally, only onehorn of the uterus is involved.
- Cats who have CEH but not pyometra may be normal onphysical examination.
- Their blood and urine tests are normal.
- Ultrasound of the abdomen is very sensitive in detecting uterine enlargement.
- Radiographs are not as useful, but being able tosee the uterus on a radiograph usually indicates the uterus is enlarged. The final diagnosis isoften not made until exploratory surgery is performed and the uterus is removed and/orbiopsied.
c) Treatment:
- There is no specific treatment for CEH.
- Theoretically, a prolongedperiod of anestrus may allow for some normalization of the endometrium.
- Progestagens mustbe avoided.
- Mibolerone is effective in inducing anestrus in cats, but is associated with seriousadverse effects.
- Maintaining queens in less than 10 hours of daylight may induce a photoperiodanestrus.
- Initial treatment for pyometra may involve intravenous fluids and antibiotics.
- SinceE. coli is the most common bacterium involved, good antibiotic choices are enrofloxacin(Baytril®), trimethoprim-sulfa (Tribrissen®), or clavulanate-amoxicillin (Clavamox®).
- It is notusually necessary to perform a culture and sensitivity test on the uterine discharge.
- Antibiotictherapy alone for pyometra is not often successful.
Douches using antiseptic or antibioticsolutions are also not effective. - One series of 183 queens (Kenney et al, 1987) with pyometrafound an 8% mortality rate, most commonly associated with a ruptured uterus and peritonitis.
Two approaches to treatment of pyometra may be taken: ovariohysterectomy and prostaglandintherapy.
i) Ovariohysterectomy provides the most consistent results as the source of theproblem is permanently removed and cats recover quickly. For queens who are not valuable toa breeding program, this is probably the best choice.
ii) Prostaglandin therapy has been themost successful treatment for open-cervix pyometra where it is desirable to preserve the futurefertility of the queen.
- Success rates for return to fertility may be as high as 86%. ProstaglandinF2 (PGF2) has been used both for pyometra and for metritis postpartum.
- The best queensfor this therapy are under 6 years of age, in good health, and have no retained fetal material ifthey are postpartum (ultrasound is very helpful in determining this).
- PGF2 therapy iscontraindicated in queens with some medical conditions such as asthma.
- PGF2 therapyshould not be used if the queen is in poor condition or is critically ill.
- Careful assessment of thepatient is critical for ruling out conditions that could preclude the use of PGF2.
- For example, inrare cases, pyometra is associated with uterine torsion, a contraindication for PGF2 treatment.
- Treatment of closed cervix pyometra should only be undertaken with caution, and only inmedically stable, young and otherwise healthy queens.
- If the cervix does not open after a fewdays of therapy, or if the queen becomes ill, she should be spayed. Only natural prostaglandinis used since a dose has not yet been established for use of synthetic prostaglandins for thispurpose in the cat.
- Queens are treated with 0.1 mg/kg of dinoprost (Lutalyse) SC, once ortwice daily for 5-7 days.
- The main purpose of the PGF2 is to cause the uterus to contract andexpel its contents.
- The luteolytic effects of PGF2 seen in other species have not beendocumented in the queen.
- If the feline CL does responds to PGF2 , it seems to take severaldays of treatment to effect luteolysis.
- Queens need to be watched closely during PGF2 therapyand may be hospitalized for the part of each day which follows administration of the drug.
- Queens must be monitored for rising fevers, abdominal pain, or other symptoms of systemicillness or rupture of the uterus (which could lead to peritonitis).
- Monitoring with radiographs orultrasound may be needed in addition to blood counts.
- The rate of complications with thistreatment is very low.
Side effects are noted often, usually within minutes of the injection, andwill be worse in the first 2 days. - The contractile effects of PGF2 on the smooth musculature ofthe myometrium, GI tract, respiratory tract, and bladder account for these reactions.
- Commonside effects include restlessness, vocalizing, panting, vomiting, diarrhea, salivation, and intensegrooming of the flanks and vulva.
- These effects usually last only a few minutes, rarely lastinglonger than 15-20 minutes.
- The reactions become less obvious with each treatment. Usually by the fifth day, little or no side effects are seen. Antibiotics should be given throughout the courseof PGF2 therapy and for some time afterward.
- Queens should be followed up by theveterinarian one and two weeks following PGF2 treatment.
- The vaginal discharge shouldchange to a clear fluid by the seventh day following treatment. This clear discharge may last forup to 10 days.
- Most cats are back to normal 2 weeks after treatment.
- If a purulent or bloodydischarge is persistent, a second course of therapy may be necessary.
- Most queens will comeback into estrus within several weeks and they should be bred at the first opportunity.
- It may bevaluable to treat the queen with antibiotics during this estrus and into the first 4 weeks of anyresulting pregnancy.
- An antibiotic safe for use in pregnancy, such as amoxicillin/clavulanic acid(Clavamox®), should be chosen.
Occasionally, a queen has a second episode of pyometraafter a pregnancy, but repeating the PGF2 treatment may still enable her to have a litter in thefuture. - After treatment with PGF2, pregnancy rates of 71-86% have been reported.
- Davidsonet al (1992) reported recurrence of pyometra within 1 year in 14% of treated cats.
- Some cats(4%) will have subclinical generalized peritonitis associated with pyometra which may contributeto ongoing problems with ill health and eventually necessitate ovariohysterectomy.
- There are noreports of successful treatment of closed pyometra in the cat although in the dog the successrate is reported to be 34%.
Feline Hyperthyroidism
by Susan Little DVM, Dipl ABVP (Feline)
Hyperthyroidism (also called thyrotoxicosis) is one of the most common diseases of the middle-aged and older cat. It is a multi-system disorder caused by an increase in the amount of thyroid hormones (T3 and T4) produced by an enlarged thyroid gland. It was first documented in cats almost 30 years ago but the cause of the disease has been elusive. Although the enlargement in the thyroid gland is caused by a tumor called an adenoma, it is non-cancerous.
The most common clinical signs of hyperthyroidism in cats include weight loss, increased appetite (although some patients have decreased appetite), vomiting, increased thirst and urination, hyperactivity, and diarrhea. The high levels of thyroid hormones can cause the development of heart disease, and these patients may have a heart murmur, difficulty breathing, high heart rate and arrhythmias.
Veterinarians will order a blood chemistry panel as well as a thyroid hormone (T4) level in cats suspected of being affected by this disease. It is important to evaluate the health of the other major organs, including the kidneys and heart in these patients. Typically, hyperthyroid cats may have elevations in their liver enzymes. Chest x-rays and cardiac ultrasound may reveal secondary hypertrophic cardiomyopathy. Generally, the cardiac changes will reverse when the hyperthyroidism is treated. In some cases, specific heart medication may be needed to stabilize cardiovascular health. In recent years, it has been recognized that many hyperthyroid cats have concurrent chronic kidney failure that is being masked by the effects of hyperthyroidism. It has also been found that treatments directed at curing hyperthyroidism in these patients could lead to a worsening of their kidney function.
Most hyperthyroid cats will have elevated levels of the thyroid hormone T4 in their bloodstream on a routine screening test. However, a small percentage of hyperthyroid cats will have normal T4 levels. If hyperthyroidism is still strongly suspected in these patients, a more sensitive test called the T3 suppression test can be performed to confirm the diagnosis. In this test, the cat is given seven oral doses of the thyroid hormone T3. Blood levels for both T3 and T4 hormones are checked before and at the end of the administration of the medication. In a normal cat, the administration of T3 hormone will cause the blood levels of T4 to drop by a negative feedback mechanism. In a hyperthyroid cat, the T4 levels will not decrease at all or will decrease very little. In this way, the veterinarian can distinguish between cats with hyperthyroidism and cats with other diseases with similar symptoms.
Once hyperthyroidism has been confirmed, there are several treatment options. They include treatment with radioactive iodine, surgical removal of the gland, and treatment with anti-thyroid medications. The initial choice of treatment is often guided by concern about the patient’s kidney function status. Some cats have detectable impairment of kidney function at the time of their diagnosis with hyperthyroidism, but many do not. It is difficult to assess kidney function accurately from routine blood testing in cats. Generally about 2/3 of the kidney function must be lost before routine blood tests will show any abnormalities. This has made it very difficult in the past to detect which cats with hyperthyroidism actually have concurrent kidney failure. However, Michigan State University has introduced a very sensitive test of kidney function in cats and dogs called the iohexol clearance test. In this test, a radiographic contrast agent called iohexol is injected intravenously and the rate at which the kidneys clear the agent from the bloodstream is measured. The test is carried out in the veterinarian’s office and a series of blood samples is sent to the MSU lab for analysis.
Since hyperthyroidism induces increases in blood pressure and blood supply to the kidneys, treating the disease will result in a drop in the blood supply to the kidneys. In a cat with kidney failure, this can cause a worsening of their kidney function in the few months after treatment for hyperthyroidism with either radioactive iodine or surgical removal of the gland. For this reason, patients with known kidney disease (either detected on routine blood work or with the iohexol clearance test) are often treated with anti-thyroid medications rather than surgery or radioactive iodine in an effort to preserve their remaining kidney function. Using medications allows the veterinarian better control over the concurrent kidney disease and may allow the patient to survive longer.
Anti-thyroid medications in current use in North America include propylthiouracil (PTU) and methimazole (TapazoleŽ). Although they are both effective in decreasing thyroid hormone levels, PTU is associated with more adverse effects than methimazole. Methimazole is better tolerated and safer for long-term use in the cat. Approximately 15% of patients will suffer from side effects when taking methimazole. These may range from poor appetite, vomiting, lethargy and skin disease to more serious problems such as bone marrow depression and liver toxicity. In most cats, the adverse effects are mild and transient and do not interfere with continued treatment. Methimazole is now available from compounding pharmacies in a transdermal gel for those patients that are difficult to pill. Transdermal gels are applied to the inner ear and the medication is absorbed across the skin. Simple precautions must be taken to avoid inadvertent absorption of the drug by the person administering the medication.
More recently, a drug called ipodate (OragraffinŽ) has been used to treat hyperthyroidism in cats. Cats with severe hyperthyroidism do not respond to treatment with ipodate as well as cats with mild disease. A study at the Animal Medical Center of 12 cats being treated with ipodate showed no side effects. However, only eight of the 12 cats showed a positive response to the drug and the beneficial effects may not last for more than a few months. This drug is most useful for short-term management of cats requiring medical treatment in preparation for surgery. Unfortunately, ipodate is expensive, generally costing about $1/day, and is difficult to obtain. This is another reason why it may not be a suitable drug for long-term treatment.
For hyperthyroid cats that are assessed with normal kidney function, surgery or radioactive iodine treatment are often recommended. Both these options provide a cure of the hyperthyroidism and avoid the need for life-long administration of medication. In areas where radioactive iodine treatment is available, it is usually the treatment of choice since this option avoids the risks of anesthesia and surgery. However, this is not a widely available treatment choice and veterinarians have become very skilled in surgical removal of the thyroid gland (thyroidectomy), making this an excellent option for treatment of hyperthyroidism in many cats.
In general, the treatment a cat receives for hyperthyroidism will depend on individual status, including heart and kidney function. Concern about kidney failure is a major determinant of the course of treatment and may eliminate radioactive iodine or surgery as an option. The advent of new kidney function testing makes it possible to assess each patient’s risk of kidney failure following treatment for hyperthyroidism.
Infertility in the Queen
.by Susan Little DVM, Dipl ABVP (Feline)
Record Keeping
- Health and breeding records are very important; breeders of pedigreed cats need a simple but complete system to record data for each breeding cat
- Individual record for each cat:
a. Call name and registered name; registration number; microchip number
b. Complete description and photo
c. Birth date
d. Sire and dam
e. Vaccination and deworming record
f. Record of major health problems and their treatment
g. Record of elective procedures (i.e. dental cleanings, spay, neuter, etc.)
- Additional records for breeding cats should include at a minimum:
a. Age at first heat
b. Record of each heat and the breeding plans.
c. Record of any health problems during the pregnancy and of anymedications administered.
d. Record of each breeding (name of tom, dates bred, number of breedings,
whether breeding was witnessed, any problems) and its outcome (i.e. pregnancy or date of return to heat)
e. Record of each pregnancy (projected due date, actual due date, number of
kittens born live/dead, any congenital defects or other problems)
f. Description of each delivery (length of time, interventions needed, etc.)
g. Dates/results of any x-rays/ultrasounds done for reproductive reasons
h. Birth weights of kittens, health problems in the neonatal period
i. Health status of each kitten at 1 and 5 years of age, if known.
Infertility
- Infertility may mean one of the following in female cats:
- Inability to be bred by a male
- Inability to conceive after successful breeding
- Inability to carry a pregnancy to term
- Cats that are difficult to breed may produce other cats who are difficult to breed!
Investigation of infertility in the queen requires:
- A complete physical exam and thorough medical history (including drugs or herbal products administered and vaccination history)
- Blood chemistries, complete blood count, urinalysis, retrovirus testing
- Vaginal cytology, serum progesterone to establish phase of estrous cycle
- Evaluation of diet, housing, show/travel stresses
- Evaluation of social interactions with other cats in cattery
- Evaluation of breeding behaviour when with male
- Evaluation of cattery environment: temperature, ventilation, available light, population density, cage design, etc.Resources for healthy indoor feline environments.
Failure to Cycle
- Immaturity: First estrus may occur any time between 4 and 21 months of age
- Senility: Queens over 8 years old may have absent or infrequent estrus cycles
- Previous ovariohysterectomy/ovariectomy: Serum luteinizing hormone (LH) will be
over 1 ng/ml in OHE/ovariectomized females due to loss of negative feedback from ovaries - True primary anestrus: Queens that fail show first estrus by 24 months of age(uncommon); evaluate karyotype for chromosomal abnormalities
- Secondary anestrus: failure to cycle, or infrequent cycles
1. Silent estrus: Normal hormonal events without behavioral estrus
• Queens that are timid, low on social scale in cattery; crowded conditions
Must differentiate from pseudopregnancy due to spontaneous ovulation
House queen with different cats (smaller group) or separately; expose to tom
2. Inadequate daylight: Indoor housing may not ensure enough hours daylight
14-16 hours artificial light necessary; equivalent to 100-watt light bulb per
13 x 13 foot space; if you can read a newspaper, it is enough light
3. Spontaneous ovulation/pseudopregnancy: Noncopulatory ovulation may be a cause of long interestrus intervals (40-50 days)
Detect with vaginal cytology and elevated serum progesterone in the absence of confirmed pregnancy
4. Intercurrent diseases/stressors: Conditions causing debilitation or prolonged ill health may affect estrous cycles; stressors such as frequent exhibition/travel, crowding, antagonistic interactions with other cats may also suppress cycles
5. Medications: Some medications may interfere with estrous cycles by suppressing gonadotropin secretion, such as corticosteroids, progestins, anabolic steroids, androgens; some antifungals such as ketoconazole can lower testosterone levels, griseofulvin could inhibit spermatogenesis
- Cabergoline (Galastop®, Boehringer Ingelheim) may be useful in inducing estrus, not well studied in the queen
- FSH can be used to induce estrus in the queen, but prolonged usage has been associated with cystic ovaries:
- Day 1: 2.0 mg, IM; Days 2 and 3: 1.0 mg, IM; Days 4 and 5: 0.5 mg, IM
Prolonged or Persistent Estrus
1. Maternal abnormalities:
• Congenital defects: persistent hymen, vaginal strictures
• Inbreeding depression: intensive inbreeding can cause subfertility, loss of vigor and reproductive capacity
• Uterine disease: cystic endometrial hyperplasia (CEH)/pyometra
a. Ultrasound uterus (uterine wall thickness, fluid accumulation)
b. Laparoscopy or laparotomy to visualize reproductive tract, biopsy/culture
uterus (for valuable queens)
c. Queens with repeated pseudopregnancies may have CEH
d. Ultrasound 18-21 days after breeding to differentiate failure to conceive from early fetal death
2. Male infertility:
breed queen to a proven sire (sired kittens within previous 6 months); check male for presence of hair ring around base of penis that can prevent intromission.
3. Breeding management issues:
• Review breeding management, videotape breedings if necessary
• Fearful queen may not breed, fear impairs hormonal events; dominant queens may require sedation to allow male to breed (best medication to use not known)
• Partner preferences and aversions are known to occur
• If queen returns to estrus less than 21 days after breeding, she did not ovulate, probably due to inadequate breedings (incomplete breedings, too few breedings)
• Check timing of breeding (too early, too late); best to breed days 2-4
• Check serum progesterone 1-2 weeks after breeding to see if ovulation occurred; ovulation is associated with serum progesterone > 2 ng/ml
4. Failure to ovulate: If breeding management issues have been ruled out as a cause, ovulation can be induced with GnRH or hCG
• Repeated treatments have been associated with immune-mediated decreases in fertility.
5. Fetal resorption/abortion:
Queen returns to estrus 60+ days after breeding.
Cystic Endometrial Hyperplasia (CEH)
Disorder of proliferative and degenerative changes in endometrium associated with aging; chronic subclinical condition; common in queens over 5 years and maiden queens over 3 years, but can be seen at any age.
Progesterone induces hyperplasia of the surface or glandular epithelium and cystic dilatation of the uterine glands; fluid in the cystic structures is usually uncontaminated, but if free in the uterus, it will support bacterial growth; progesterone also inhibits the local immune response and decreases myometrial contractility.
Repeated pseudopregnancies may predispose some queens to CEH; progestins used to control estrus also a risk factor.
Diagnosis: queen is not ill but fails to conceive or has small litters; ultrasound maydetect thickening of uterus; definitive diagnosis only with uterine biopsy.
No treatment for uncomplicated CEH; endometritis (CEH plus bacterial infection)may respond to prolonged antibiotic treatment but can progress to pyometra.
Pyometra
Severe endometrial infection with accumulation of pus in uterus
Typically occurs following an estrus, when bacteria from vagina invade uterus through open cervix; usually associated with CEH
Vagina normally has bacteria present; vaginal cultures therefore hard to interpret; most common bacteria are E. coli and Streptococcus, Staphylococcus, etc.
Clinical signs: Vulvar discharge (if cervix open), depression, dehydration, anorexia, fever, weight loss, distended abdomen
Diagnosis: Increased white blood cell count, enlarged uterus on x-rays or ultrasound.
Treatment: IV fluids may be needed, antibiotics (fluoroquinolones, amoxicillin- clavulanate) plus ovariohysterectomy (OHE) or prostaglandin therapy for valuable breeding queens.
- Antibiotics alone, vaginal douches not very effective.
- Prostaglandin therapy: best for open-cervix; complication rate is low!
- Candidate queens are under 6 years, in good health (no asthma), no retained fetal.
- The material or live fetuses, no complications (i.e. uterine torsion)
- Prostaglandin F2α, dinoprost (Lutalyse ®, Pharmacia & Upjohn) treatment: queenmay or may not be hospitalized; different dosing options:
High dose: 0.1 to 0.25 mg/kg SC twice daily for 5 to 7 days
Low dose: 0.02 to 0.05 mg/kg SC 4-6 times daily for 5 to 10 days
- Treatment may cause cervix to open, uterus to contract, may cause lysis of CL
- Monitor for: rising fever, abdominal pain, systemic illness, uterine rupture
- Assess success of treatment by monitoring white blood cell counts, ultrasound
- Side effects common at higher doses, especially first day: restlessness, vocalizing,panting, vomiting, diarrhea, salivation, intense grooming of flanks and vulva; lasts 15-20 min; side effects uncommon at low doses (often only a bit of salivation)
- Re-examine cat 1 and 2 weeks post-treatment: clear vulvar discharge by day 7, normal by 14 days; if bloody or purulent discharge persists, treat again.
- Breed at next heat! Fertility rates after treatment are good (80% or better)
- Adjunctive therapy:
Prolonged oral antibiotic therapy (4-6 weeks)
Cabergoline (Galastop®, Boehringer Ingelheim): 5 μg/kg, PO, 5 days; prolactin inhibitor, luteolytic, no side effects Aglepristone (Alizine®, Virbac): progesterone receptor antagonist, 10-15 mg/kg, SC, 2 days in a row.
Gastrointestinal Helminths in Cats
High Prevalence of Covert Infection With Gastrointestinal Helminths in Cats
Susan Little, DVM, PhD, DACVM-Parasit ; Chris Adolph, DVM, MS ; Kathryn Downie, DVM ; Tim Snider, DVM, PhD, DACVP, ; Mason Reichard, MS, PhD
J Am Anim Hosp Assoc (2015) 51 (6): 359–364.
https://doi.org/10.5326/JAAHA-MS-6221
Fecal flotation is routinely used to identify feline helminth infections in clinical practice, but it is known to have limitations of sensitivity, particularly for cestodes.
To determine the prevalence of helminths in a contemporary population of cats and evaluate the ability of fecal flotation to detect these infections, helminths were recovered from intestinal tracts removed from 116 adult cats humanely euthanized by an animal control shelter in northeastern Oklahoma.
Results were compared to those of fecal flotation performed using both passive and centrifugal techniques.
Helminths were identified in 78/116 (67.2%) cats, including Toxocara cati (48/116; 41.4%), Ancylostoma tubaeforme (8/116; 6.9%), Dipylidium caninum (40/116; 34.5%), and Taenia taeniaeformis (30/116; 25.9%). Cats with T. cati were significantly more likely to harbor T. taeniaeformis (P = .001) than cats without ascarids.
Centrifugal fecal flotation with sugar solution identified 37/48 (77.1%) T. cati infections, 8/30 (26.7%) T. taeniaeformis infections, and no D. caninum infections. Proglottids were detected on external examination in 19.0% (12/63) of cats with cestodes. Cestodes were present in over half of the cats examined in this study, but the majority of these infections were not evident by the detection of external proglottids or recovery of Skip to Main Content characteristic stages on fecal flotation.
New ideas about old viruses
by Susan Little DVM, Dipl ABVP (Feline)
American Association of Feline Practitioners (AAFP) is excited to announce a long-awaited update to the 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines1 in this issue of JFMS.This 2020 update was written by an international Advisory Panel composed of feline specialist veterinarians in private practice (SL, GO, KSD), a veterinarian board certified in internal medicine and shelter medicine (JL), and three European experts who have provided much original research on feline retroviruses (KH, RHL, MH). While these guidelines were primarily written with the North American veterinarian in mind, the international scope of the contributions means that we are honored to have endorsement from the International Society of Feline Medicine (icatcare.org/veterinary/isfm).
The last AAFP retrovirus guidelines were published 12 years ago, and so the 2020 guidelines represent a major update on the pathogenesis, diagnosis, prevention and treatment of feline retrovirus infections. Much has changed in the intervening years, especially in our understanding of feline leukemia virus (FeLV) infection. The outcomes of infection with FeLV have been redefined, and new test methodologies are available. There is now recognition that the clinical expression and prognosis for FeLV may change over time relative to the cat’s current immune status and resulting levels of virus in circulation. This ushers in an era in which quantitative FeLV testing may be used to better predict outcomes.
For feline immunodeficiency virus (FIV), new research has improved our knowledge of the effect of the virus on morbidity and mortality, and shows the outcome of infection may depend, in part, on the cat’s environment. Point-of-care tests have been identified that distinguish between FIV-infected cats and FIV-vaccinated cats.
Ideas about determining the retrovirus infection status of a cat have also changed. Since cats are tested under various circumstances and for various reasons, a single testing protocol is difficult to recommend for all cats. In these guidelines, we present some practical options. A further difficulty is that a single test at a single point in time may not be sufficient to determine a cat’s infection status, especially for FeLV. However, one thing has not changed: the most important measure for the control of FeLV and FIV remains identification of infected cats. These guidelines address an emerging trend in which screening for FeLV and FIV is increasingly shifting from animal shelters, where cats are adopted, to veterinary practices, where animals receive comprehensive care.
Cat owners and caregivers play an important role in the identification and treatment of infected cats and prevention of infection. Adoption programs for infected cats are growing in popularity, necessitating a new area of focus for veterinarians in both shelters and practice. A client brochure (pictured) accompanies these guidelines and can be downloaded from the AAFP (catvets.com/guidelines/client-brochures) and JFMS (jfms.com) websites. The Advisory Panel hopes these guidelines and the client brochure will be of benefit to veterinarians, whether in private practice, shelter medicine or educational institutions.
2020 Advisory Panel Co-Chairs
Feline Infectious Peritonitis
by Susan Little DVM, Dipl ABVP (Feline)
One of the most poorly understood and enigmatic feline viruses is the feline coronavirus -the virus responsible for feline infectious peritonitis (FIP). In fact, the virus causing FIP, feline coronavirus (FCOV), is very common among cats, especially in multi-cat households, catteries, and shelters. Multi-cat situations of several years’ duration will likely have a brush with FIP. It is no cause for either fear or ostracization. It is, however, a reason to make efforts to understand this disease and its means of control.
While the first description of feline infectious peritonitis was reported by Dr. Jean Holzworth in 1963, there are reports of clinical cases that are likely FIP going back to 1914. Even though we have known about this virus for a long time, we know frustratingly little about it. Research over the past 10 years is slowly shedding more light on this ever-present feline health problem. This article is designed to present some of the newer information and change some of the older ideas still found in print and other media.
Feline coronavirus operates differently from any other feline virus in several important ways:
a) Systemic antibodies have no protective function for the cat and may play a role in the disease FIP itself
b) Antibody titers are meaningless for diagnosis of FIP or prognosis; detection of the feline coronavirus is also not diagnostic, and currently no test exists that can distinguish the virus of FIP from the enteric form of FCOV c) A vaccine is available, but there is no consensus on its efficacy or safety.
First, some notes on terminology. FIP is the term for clinical disease associated with feline coronavirus infection. The common benign form of feline coronavirus is referred to as FECV (feline enteric coronavirus). When FECV has mutated into a disease-causing form, it is then referred to as FIPV (feline infectious peritonitis virus). Feline coronaviruses in general are referred to as FCOV.
FECV is a very common, highly infectious feline virus. It belongs to the genus Coronavirus, which has members that infect other species (man, swine, cattle, birds, dogs). The majority of cats with FECV (about 90% or more) remain healthy. But in a small number of cases, FECV infection is the first step in a chain of events leading to FIP.
This happens because coronaviruses are made of large numbers of nucleotides, the basic unit of genetic material, and they are very prone to mutations. As a virus reproduces itself, errors are made in copying these nucleotides. The more nucleotides, the more errors are possible. While most of these errors are harmless, some will have the effect of giving FECV the ability to cause disease. These mutant FECV strains are called FIPV. The precise mutation or mutations leading to disease development have not been clarified. Several possibilities exist, with
Member Combined Federal Campaign #10321
changes in several genes having been observed in FIPV, but consensus as to which specific mutations are responsible for causing the lethal disease has not been reached.
Recent research has shown that mutant FECVs arise within an individual cat. Thus, we now know that the vast majority of cats do not «catch» FIP, but they develop it themselves from their own mutant FECV. Non-pathogenic FECV lives and replicates within the cells of the intestinal tract and may be shed into the feces.
But FIPV (the mutant form of FECV) has developed the ability to live and replicate within a type of white blood cell of the immune system that is called a monocyte (when in blood) and a macrophage (when in tissues).
This ability of the virus to replicate to high levels in monocytes and macrophages is critical to the development of FIP. FIPV are able to spread throughout the cat’s body, and are no longer localized in the intestinal tract and so are rarely shed into feces.
Transmission of FIP from cat to cat is considered to be rare. This fact has caused leading FIP researchers to state that cats that are ill with FIP are unlikely to be a risk to other cats and thus do not need to be isolated.
A recent investigation identified mutations in FCOV found in tissues other than the intestines. These mutations, occurring in one gene (the 3c gene) were never identified in viruses found in the intestines.
The investigators speculate that this gene may be required for efficient intestinal replication, and its mutation results in loss of this ability. Thus, this mutation may lead to increased virulence of the virus but prevent its replication in the gut, thereby preventing shedding in feces of the affected cat.
A kitten ill with the dry form of FIP.
It has been estimated that in multi-cat households where FECV has been introduced, 80%-90% of all the cats will be infected. In the general cat population, infection rates may reach 30%-40%. Multi-cat situations and catteries are especially likely to be FECV-positive since traffic of cats and kittens in and out of the establishment is common. However, the incidence of cases of FIP is quite low in comparison.
Generally, most of these establishments experience far less than 10% losses to FIP over the years. Rare instances have been documented in which an apparent epidemic of FIP is associated with mortality rates of over 10% in a short period of time. One possible factor in these epidemics is the shedding of virulent virus, an uncommon situation. Usually, losses are sporadic and unpredictable. The peak ages for losses to FIP are from 6 months to 2 years old (with the highest incidence at 10 months of age).
Age-associated immunity to FIP appears to be possible, although cases of FIP have been reported in elderly cats. Transmission of FIP from a queen to her unborn kittens has not been shown to occur, but transmission of FCOV from queen to kittens is common.
What are the factors that predispose a small percentage of cats with FECV to the development of FIP? Research is currently trying to find more answers to this question, but some facts are becoming clear. Dr. Janet Foley and Dr. Niels Pedersen of the University of California at Davis have identified three key risk factors: genetic susceptibility, the presence of chronic FECV shedders, and cat-dense environments that favor the spread of FECV.
Member Combined Federal Campaign #10321
Drs. Foley and Pedersen identified a genetic predisposition to the development of FIP in 1996. They examined pedigree and health data from 10 generations of cats in several purebred catteries and found that the heritability of susceptibility to FIP could be very high (about 50%). It is likely a polygenetic trait rather than a simple dominant or recessive mode of inheritance. Inbreeding, by itself, is not a risk factor. Several breeds, including the Bengal, Birman, and Himalayan, are more likely to develop FIP. In addition, susceptibility along familial lines has also been documented. Selecting for overall disease resistance is a helpful tool for breeders.
The disease itself is primarily mediated by the immune response to the virus, rather than the virus itself. The mutant virus replicates efficiently in monocytes and macrophages throughout the body. In cats that develop FIP, the virus is not effectively cleared for reasons that remain unknown. These animals mount a good antibody response, but this is ineffective at clearing the virus – antibodies target mainly the viruses outside the infected cells.
Effective viral clearance requires cell-mediated immunity, in which cells of the immune system target infected cells, thus eliminating these «virus factories.» Why some cats mount this ineffective response is the subject of much investigation, and as suggested above, likely has a genetic component.
The probable defect in immunity to FIP is in cell-mediated immunity. This type of immunity is important in defense against pathogens that replicate within a cell (as opposed to those that colonize extracellular spaces). Therefore cats that are susceptible to FIP are also likely susceptible to some other infections as well, especially fungal and viral infections. This finding gives breeders the ability to achieve success in reducing the risk of FIP by using pedigree analysis to select breeding cats from family backgrounds that have strong resistance to FIP and other infectious diseases.
Thus, to produce FIP, it takes not only the mutant virus, but a cat predisposed to mount an ineffective immune response to the virus – the «right» virus and the «right» cat. This is why FIP is uncommon, though infection with FCOV is widespread among cats.
The lesions of FIP arise from the immune response to the virus. Infected cells accumulate along the walls of blood vessels, and even migrate into the surrounding tissue. The inappropriate antibody mediated response by the immune system leads to tissue damage at these sites through a variety of mechanisms. So-called «innocent bystanders» – uninfected cells – are often killed in the process.
This leads to an intense inflammatory response that may be widespread along the blood vessels (effusive FIP) or it may be localized to specific tissues, such as the eye, or kidney (non-effusive FIP). Because this antibody response is ineffective at clearing the infection, the virus continues to replicate and accumulate, as does the inflammatory response. This progresses to multi-organ failure and inevitably death.
There are two forms of FIP. The effusive (or wet) form is characterized by accumulation of fluid in the chest or abdomen. This fluid is high in protein content and is often a yellow color.
The yellow fluid found in the abdomen of a kitten that died from wet FIP.
The non-effusive (or dry) form of FIP is characterized by inflammatory lesions called pyogranulomas that can be found in almost any organ of the body, including the nervous system.
Member Combined Federal Campaign #10321
common to both effusive and non-effusive forms of FIP include loss of appetite, weight loss, lethargy, and a fluctuating fever that is not responsive to antibiotics. Cats with the effusive form may develop a swollen abdomen or difficulty breathing because of fluid accumulation.
A kitten with the distended abdomen typical of the wet form of FIP.
As with so many aspects of FIP, testing remains problematic. To date, there is no way to screen healthy cats for the risk of developing FIP. Antibody titers are poorly correlated with risk of FIP and should not be used to screen healthy cats.
As well as problems with interpretation of these antibody tests, there are problems with laboratory quality control. In addition, cats that have been vaccinated with some types of vaccines may test positive on coronavirus antibody tests due to cross-reaction between components of the cell culture used to produce the vaccine and test system components. It remains true that a negative antibody titer does not rule out FIP. Neither does a positive antibody titer rule in FIP as a diagnosis, even if that titer is very high.
Finding the virus itself in a cat is also not diagnostic for FIP. As stated above, the virus is common, and can be found in the blood of infected yet healthy cats. There are now DNA-based blood tests offered by a few labs that are purported to be FIP-specific. However, there are no published studies that have identified the genetic difference between FECV and FIPV, and most experts doubt the validity of these tests.
A newer test from Auburn University, called the mRNA test for coronavirus, detects virus actively replicating outside the intestinal tract. This approach shows promise as an aid in the diagnosis of FIP, as efficient replication outside the gut occurs in FIP. But the fact remains that we have no screening test for FIP in well cats. Neither do we have a foolproof way to diagnose FIP in a sick cat. And always bear in mind that no current test can distinguish the FCOV that causes little or no disease from the FCOV that leads to FIP.
Dr. Andrew Sparkes and his colleagues at the University of Bristol, England, have suggested that combining several test results (e.g., globulin levels, lymphocyte counts) with clinical findings and antibody titer can help rule in or rule out FIP with some degree of certainty. In fact, combining test results that correlate with FIP (building the diagnostic «brick wall” for FIP, with each brick a separate test result), as well as ruling out other diseases, remains the only reliable means of antemortem diagnosis. The gold standard is still examination of affected tissue using a biopsy or findings at necropsy.
Unfortunately, there is no known effective treatment for cats with FIP. Treatments are aimed at palliative care and patient comfort. Drugs that suppress the immune system response to FIPV (such as prednisone) may temporarily stabilize patients. Newer therapies, such as recombinant feline interferon and pentoxifylline, have shown some limited success in a small number of patients but require more investigation before they can be recommended. Feline interferon is not available in all countries.
Member Combined Federal Campaign #10321
Recently, a new drug tested in three cats with the dry form of FIP demonstrated efficacy in prolonging life and alleviating signs. The drug, a polyprenyl immunostimulant, is an investigatory veterinary biologic and acts on the lymphocytes responsible for effective cell-mediated immunity. In this study, two cats with FIP were still alive two years after diagnosis, while one cat survived 14 months. Further studies are under way to assess its potential for FIP treatment.
Sadly, the majority of patients eventually deteriorate to the point where humane euthanasia is chosen.
So how can FIP be controlled? In addition to efforts in breeding resistant animals, preventing or reducing the incidence of FCOV infection is important. FECV is spread primarily by the fecal-oral route
degree, through saliva or respiratory droplets. The virus can persist in the environment in dried feces on cat litter for 3 to 7 weeks, so scrupulous cleaning of cages and litter pans is important to reduce the amount of virus in the environment. Fortunately, FECV is susceptible to most common disinfectants and detergents. It is important to have adequate numbers of litter pans. Litter pans should be kept away from food bowls and spilled litter should be regularly vacuumed up from the floor.
Research has shown that there are two main patterns that occur with FECV infection. Most cats will become infected and recover, but will not be immune. They are susceptible to reinfection the next time they contact the virus. A small number of cats become infected but do not recover. They become persistent shedders of FECV in the cattery or shelter and are the source of reinfection for the other cats although they may never become ill.
Therefore, the key to eliminating FECV (and thus the risk of FIP) in a cattery or other multi-cat establishment would be the identification and removal of chronic shedders. Currently, however, there is no easy way to determine which cats in a multi-cat environment are persistent shedders.
The traditional antibody titer for FECV cannot reliably be used to determine which cats are chronic shedders. The most effective and practical tool is PCR analysis of feces for the presence of FECV, a test that is not yet widely available. Because shedding of the virus may be intermittent, testing of multiple samples over time is required. Dr. Hans Lutz at the University of Utrecht has shown that PCR on four fecal swabs taken at one-week intervals can determine the coronavirus-shedding status of a cat.
In addition to selecting disease-resistant breeding stock, breeders, shelter managers, and other multi cat households can initiate husbandry practices that discourage the spread of FECV and development of FIP. Cat-dense environments favor the transmission of the highly contagious FECV. Dr. Diane Addie of the University of Glasgow, Scotland, recommends that the ideal way to house cats in catteries is individually.
However, since this is not always possible, she recommends that they be kept in stable groups of no more than 3 or 4. Kittens should remain in groups of similar ages and not be mixed with adults in the cattery. Any measures that reduce environmental and social stress in the cattery population will have a beneficial effect.
Dr. Addie has also described a method for early weaning and isolation of kittens born to FECV-positive queens designed to prevent infection of the kittens. It involves rigorous barrier nursing techniques to prevent the spread of the highly contagious FECV, and so is not for every breeder or cattery.
The procedure involves first isolating the pregnant queen in a separate area to have the kittens. When they are 5-6 weeks old (at the time when their maternal immunity to FECV is waning), the kittens are removed from the queen and isolated by themselves. Some of the difficulties with this method involve the strict infection control procedures needed and possible difficulties in socializing kittens.
It also been reported by breeders that some early-weaned kittens may have behavioral problems, such as inappropriate suckling on littermates. When properly practiced, not only can FECV-negative kittens be produced, but the kittens are often less prone to respiratory diseases and other common kitten ailments.
Member Combined Federal Campaign #10321
Probably one of the most controversial areas in any discussion of FIP is Primucell FIP, the vaccine made by Pfizer Animal Health, available since 1991.
The vaccine is a modified-live temperature sensitive viral mutant licensed for intranasal use in cats at least 16 weeks of age. The manufacturer recommends annual revaccination although no duration of immunity studies are available. The vaccine stimulates local immunity and may also produce an antibody titer. Evaluation of the risks and benefits associated with this vaccine is a difficult venture and has engendered much controversy.
Since FIP is a severe and fatal disease, the safety of any vaccine is a paramount consideration. Dr. Fred Scott of the Cornell Feline Health Center concluded in a recently published paper, that the risks associated with the Primucell FIP vaccine are minimal in most situations. He notes that the vaccine has been in use for many years with no increase in the incidence of FIP.
Troubling reports of a phenomenon called «antibody-dependent enhancement» (ADE) of infection arose from several labs, where cats vaccinated with FIP vaccines and challenged experimentally with virus developed accelerated disease instead of being protected. It is not known whether the phenomenon of ADE occurs in the real world and there is no easy way to find out. If it does occur, it is likely an uncommon event, but the possibility remains troubling.
On the other side of the issue, the benefits of the Primucell FIP vaccine appear to be small. The best reported efficacy for the vaccine is seen when FCOV negative cats at least 16 weeks old were vaccinated twice (3 weeks apart), in a study by Dr. Nancy Reeves published in 1995. In this study, FCOV antibody-negative cats were vaccinated before entering a large cat shelter where FIP was endemic. The vaccinated cats experienced a significantly lower mortality rate than unvaccinated cats. The efficacy of the vaccination was calculated to be 75% (preventable fraction).
In catteries where FIP is endemic, studies have shown the vaccine had no effect on the incidence of disease. One reason may be that most kittens in catteries are infected between 6 and 10 weeks of age, long before the 16 weeks of age the vaccine is licensed for.
Once a cat is infected with FCOV, the vaccine has no benefit. Some cattery owners have been using the vaccine at ages younger than 16 weeks to get around this problem.
Dr. Johnny Hoskins has outlined a vaccination protocol for catteries experiencing FIP losses in kittens less than 16 weeks of age. He recommends giving the vaccine at 9, 13 and 17 weeks with annual revaccination afterward. Use of this protocol must be made with the knowledge that no controlled studies have been done on kittens under 16 weeks of age and that this is an off-label use. It would appear that the use of the vaccine according to the manufacturer’s directions is limited to the vaccination of FCOV antibody-negative cats entering high-risk situations, such as catteries and shelters.
In conclusion, much has been discovered about FIP, but the picture is far from complete. As more is discovered, there will be improvement in our understanding of, our ability to diagnose, and our ability to treat and control this terrible disease.
Please Note: The Winn Feline Foundation provides the feline health information on this site as a service to the public.
Diagnosis and treatment of specific conditions should always be in consultation with one’s own veterinarian.
Winn Feline Foundation is a non-profit organization (501)(C)(3) established by the Cat Fanciers’ Association
Non-obstructive Feline Lower Urinary Tract Diseas
by Susan Little DVM, Dipl ABVP (Feline)
It has been estimated that up to 1.5% of all cats in the United States suffer from a lower urinary tract disorder. One survey showed that up to 9% of cats taken to veterinarians in Japan also suffer from these problems. Cats can develop a number of problems related to the bladder and urethra. Commonly used terms are FUS (feline urologic syndrome) and FLUTD (feline lower urinary tract disease).
The confusion over names reflects the confusing and changing nature of these problems in cats. As long as 70 years ago, signs of lower urinary tract disease in cats were commonly seen by veterinarians. Today, these remain some of the most challenging and frustrating problems in feline medicine.
A common term used in describing some of these conditions is cystitis, which means inflammation of the bladder. Urethritis means inflammation of the urethra, the tube leading from the bladder to void urine outside the body. There are several sub-types of identifiable problems associated with lower urinary tract disease in cats.
They include: idiopathic cystitis (also sometimes called interstitial cystitis), bladder stones (uroliths): covered in a separate article, anatomical defects, behavioral abnormalities, urethritis, bacterial infections (bacterial cystitis) – neoplasia (tumors of the bladder), and crystalluria (presence of crystals in the urine).
Regardless of the cause, there are common symptoms that may be seen in cats with any lower urinary tract disease.
They include: hematuria (blood in the urine), dysuria (difficulty passing urine), pollakiuria (increased frequency of urination), and urinating out of the litter box.
While many of the conditions discussed in this article can also lead to partial or total urethral obstruction, the typical patient is presented without an obstruction.
When a cat is presented to the veterinarian for possible lower urinary tract disease, the vet will first determine if a urinary obstruction exists by palpating the bladder.
This article deals with non-obstructive cases, which are the most common type. (More information on urethral obstruction is provided in the article on Feline Bladder Stones and Urinary Obstructions.)
Evaluation of a cat with signs of lower urinary tract disease starts with taking the patient’s history and a good physical examination.
The bladder is palpated for thickness, fullness, and possible bladder stones. Since many cats urinate in inappropriate places in response to behavioral problems, one of the first tasks is to determine if a medical or behavioral problem exists. At the very least, a urinalysis is usually performed. In other cases, more tests must be done, such as blood tests, X-rays or ultrasound.
Urine samples are checked for the urine pH, presence of blood or infection, the specific gravity (a measure of concentration) and for crystals.
Recent studies have found that taking a cat outside its home for a trip, such as to the veterinarian, can lead to rapid changes in the urine pH. In some cases, therefore, your veterinarian may recommend ways to collect a urine sample at home before the cat is brought into the clinic.
Determining the significance of crystals in the urine of a cat can be problematic. It is known that many normal cats have small numbers of crystals in their urine.
On the other hand, up to 50% of cats that have bladder stones may have no evidence of crystals at all on their urine test. Generally, only moderate to severe cases of crystalluria should be considered significant and treated.
Such cases are usually of dietary origin, and identifying the type of crystal is necessary to pick the appropriate therapeutic diet. Common types of crystals in feline urine are struvite (magnesium ammonium phosphate) and calcium oxalate.
These crystal types each require different types of dietary therapy. Feeding only canned diets to cats diagnosed with urinary crystals may help to reduce the risk of recurrence.
If the cause of the cat’s symptoms has not been identified with a urinalysis, other testing may be recommended. A urine culture is used to check for bacterial infection, although this accounts for probably less than 2% of cases.
Blood tests are done to check for diseases that can also influence the urinary tract, such as diabetes, hyperthyroidism and kidney failure. X-rays or ultrasound are used to identify bladder uroliths (stones), anatomic defects, or tumors.
Therapy of lower urinary tract disease is directed against the underlying factors discovered during testing. Significant crystalluria is treated by prescribing the appropriate therapeutic canned diet. If a positive bacterial culture has been found, antibiotics are prescribed on the basis of the sensitivity results. Anatomical defects may require surgical repair.
Bladder tumors are uncommon in cats, and may require one of the various treatments used for cancers. Therapy for bladder uroliths is covered in a separate article. Various medications may also be used to decrease pain, to help the bladder contract effectively, or to decrease any irritation or spasms in the urethra.
Increasingly, many cats are found to have moderate to severe signs of lower urinary tract disease without having a readily identifiable cause. They typically have blood in their urine, but no bacterial infection. They do not have tumors, anatomical defects, or bladder stones. They have no other diseases causing their symptoms. The term interstitial cystitis, borrowed from human medicine, has been used to describe these cats. An alternate term is idiopathic cystitis. The only way to definitively confirm the diagnosis is by cystoscopic examination or biopsy of the bladder, so many cases are tentatively diagnosed after all other conditions have been ruled out.
Therapy of interstitial cystitis is currently evolving. Reduction of stress in the cat’s environment is very important. Food and water stations, as well as the litter box, should be placed in quiet areas away from sources of noise such as appliances, fans, air ducts and away from other pets. These cats may prefer canned foods and clumping, unscented types of litter. When trying a new cat litter, it is important to add a second litter box with the new litter to ensure it is accepted before discontinuing the previous litter type. It can also be helpful to provide safe areas for these cats, such as secluded sleep areas. Increasing vertical space by using tall and sturdy cat trees can allow the cat to reach an area where it will be undisturbed.
A synthetic cat pheromone called Feliway® has been produced and is available commercially through veterinarians and pet stores.
This product is able to exert a calming effect on some cats that may help reduce inappropriate elimination behaviors. In severe, recurrent cases, a drug called amitriptyline (Elavil®) may be prescribed by the veterinarian.
This drug is used to decrease the pain associated with episodes of cystitis and to decrease the inflammation in the bladder lining. Side effects, such as sleepiness, retention of urine, bladder stone formation, weight gain, and reduced grooming, may be seen in some patients. However, amitriptyline has been prescribed for cats for many years and the overall rate of side effects appears to be small and the degree is usually mild. Most cats tolerate the medication well.
Corticosteroids, such as prednisone, are also sometimes used to decrease inflammation, and other types of drugs, such as pentosan polysulfate or glucosamine, are used to help repair the lining of the bladder.
However, evidence for the effectiveness of these therapies is lacking. No matter what type of therapy is used, it is important to decrease any stress factors as much as possible. No one therapy to date has been found to help all cats, and we currently are only able to aim for control of the problem and not a cure.
Hopefully, ongoing research and drug trials will provide some better therapies in the future.
For more information: The Indoor Cat Initiative (Idiopathic/Interstitial Cystitis in Cats) http://vet.osu.edu/indoorcat
Please Note: The Winn Feline Foundation provides the feline health information on this site as a service to the public. Diagnosis and treatment of specific conditions should always be in consultation with one’s own veterinarian. The Winn Feline Foundation disclaims all warranties and liability related to the veterinary information provided on this site.
Understanding genetics: why should vets care?
by Susan Little DVM, Dipl ABVP (Feline)
The genomes of hundreds of dogs have been sequenced, and the genomes of pedigreed and non-pedigreed cats are in the pipeline via the 99 Lives Cat Genome Sequencing initiative.1 Most veterinary colleges teach genetics in their core curriculum. But why should a feline practitioner care about genetics?
We live in an age where everything ‘is in our dNA’, whether you’re a Sony high definition TV or a Jaguar automobile. A growing proportion of the general public understands that our good genetic make-up can be squandered by poor lifestyle andbad habits; but also,some people whohave smoked a packof unfiltered LuckyStrikes every day canlive to the age of 84.
Genetic testingcan help us humansanticipate ourhealth risk factors.it can do the samefor cats.We can alreadyanticipate specific health concerns in feline patients using genetic testing for some diseases. dNA is the basis of life and the foundation of organismal biology. if you accept comparative anatomy, you must accept comparative genetics, as the majority of genes are the same in all mammals.
Gene timing and regulation may vary among species, but only a few hundred genes out of 20,000 or so are specific to a cat. Some defects, like polycystic kidney disease, are obvious problems with a genetic basis that can be readily appreciated by cat owners and veterinarians alike. But there are other diseases, such as feline infectious peritonitis, lymphoma, asthma, diabetes and inflammatory bowel disease, where the importance of genetic predisposition is less intuitive. But you can bet your bottom dollar that genetics underpins susceptibility to almost all diseases, including predispositions to infectious diseases. Novel genetic mutations are the cause of many sporadic, idiopathic conditions, including the maladies that affect the majority of the cat population, the non- pedigreed cat.
The trend for genetic testing and whole- genome sequencing will develop significantly as the standard-of-care for feline patients continues to improve, and cats will continue to grow in popularity. in the coming years, veterinarians will be performing whole- genome screens of some kind as a routine component of a diagnostic investigation.
Whether for tumors or other diseases, the dNA results will be what routinely directs future therapies.
Via the internet, anybody can just ‘Google it’ and question authority as if they are an expert in the field (the know-it-all client!). And, we all know the internet was made for cats – they are the internet stars! indeed, everything you need to know about genetics, you can learn from your cat; thus, cat owners tend to be fairly savvy when it comes to inheritance patterns.
For the veterinarian to be perceived as an expert in the eyes of the client, they must have a solid genetics foundation. Resources are available for refresher courses.
2 Feline practitioners should accept an ‘educated’ public and must be able to explain clearly, in a ‘non-scientific’ manner, how genetic methods can identify and control inherited diseases, as well as produce desirable features and prevent undesirable features.
Knowledge of genetics is power. Cats can be bred in a manner to prevent illnesses, eliminating problems before they exist.
Genetics can be used to eradicate a disease, never to be seen again (until a new mutation occurs). However, the veterinarian may not have the requisite skill set to ‘do it by themselves’. Fortunately, feline genomics is a science where robust international collaborations based on mutual trust and respect represent the cornerstone of obtaining successful outcomes for our feline patients. in genomics, working together has solved the genetic basis for familial hypokalemia in the Burmese cat and a variety of other conditions, albeit getting all the players on the same page is rather like herding cats.
The power of genomics is contingent upon finding and sampling sufficient affected, carrier and normal cats of whatever disease you are working on, breed-related or not. Without doubt, the veterinarian has a key role in helping feline genomics leap forward into the future.
It’s a terrific time to develop a better understanding of modern genomic approaches to feline health, and to be part of cutting-edge approaches to unravelling important feline diseases. As discussed in a clinical review on pages 203–219 of this issue – the first of a handful of JFMS reviews devoted to feline genetics in the coming months – over 70 different genetic variants have been identified in cats that confer coat colors, fur types, morphological attributes, blood type and diseases. Hundreds of thousands of humans have had their whole genome sequenced to improve their own health or to help identify health-related dNA variants in their children.
Let’s work together to bring our beloved cats to the same state-of-the-art healthcare!
- Boyd R Jones, Massey University, NewZealand
- Susan Little, Bytown Cat Hospital, ottawa,Canada
- Leslie A Lyons,* University of Missouri, USA
- Richard Malik, University of Sydney,Australia
- Frank Nicholas, University of Sydney,Australia
- Dennis P O’Brien, University of Missouri,USA
- Niels C Pedersen, University of California, davis, USA
*Corresponding author, Email: lalyons@ucdavis.edu
Resources
http://felinegenetics.missouri.edu/ninety-nine-lives
Nicholas FW. introduction to veterinary genetics.
3rd ed. oxford: Wiley-Blackwell, 2010.
Cryptorchidism in Pedigreed and Non-Pedigreed Cats
Susan Little, DVM, DABVP (Feline)
Bytown Cat Hospital
Ottawa, Ontario, Canada
Case reports of cryptorchidism in cats have appeared occasionally in the veterinary literature, but there is little detailed incidence data available on cryptorchidism in large populations of cats presented for castration. As well, scant data are available on the incidence of cryptorchidism in various pedigreed breeds.
Records of 4,140 cats presented for castration to two feline-only veterinary hospitals over a 10-1/2 year period (January 1993 to June 2003) were examined. Seventy-two cats (1.7%) were identified as cryptorchid. This incidence is similar to the findings in other studies (range 1.3% to 1.9%) of large numbers of cats, but lower than that reported in one smaller study (3.8%). Incidence rates reported in dogs in recent studies vary from 2.6% to 6.8%.
Three cats (< 0.08%) in the current study were identified as monorchid (two Persian/Himalayans, one random bred). This finding is lower than the incidence of monorchidism identified in an earlier study (0.15%) that involved a smaller number of cats.
Of the 4,140 cats presented for castration, 437 (10.5%) were pedigreed cats, representing 22 breeds. The incidence of cryptorchidism in pedigreed cats was 6.2%, significantly higher than that for non-pedigreed cats (1.3%). The incidence of cryptorchidism in pedigreed dogs is also higher than that for random bred dogs. Eight cat breeds were represented by 10 or more individuals (Table 1).
Table 1. Incidence of cryptorchidism in eight pedigreed breeds
|
Breed |
# presented for castration |
# cryptorchid (%) |
|
Abyssinian/Somali |
15 |
1 (6.7) |
|
Balinese |
10 |
0 |
|
British Shorthair |
19 |
0 |
|
Burmese |
19 |
2 (10.5) |
|
Maine Coon |
16 |
2 (12.5) |
|
Persian/Himalayan |
135 |
14 (10.4) |
|
Ragdoll |
16 |
3 (18.75) |
|
Siamese |
158 |
4 (2.5) |
The highest incidence of cryptorchidism was found in the Ragdoll breed (18.75%), although only a small number of Ragdoll cats (16) were included. Previous studies have found the incidence of cryptorchidism in the Persian breed to be as high as 29%. In the current study, which included a larger number of Persian/Himalayan cats than previous reports, the incidence in the breed was 10.4%.
The location of the retained testicle(s) was known for 62 of the 72 cryptorchid cats. Most cats (88.7%) were unilateral cryptorchids. The most common configuration was the unilateral, inguinal cryptorchid (51.6%). Right unilateral and left unilateral cryptorchids were similarly represented. Previous reports have shown conflicting data on the most common location of the unilaterally retained testicle. In the unilateral cryptorchids of this report, the retained testicle was significantly more likely to be inguinal. Only 7 of the 61 cats (11.3%) were bilateral cryptorchids; 2 (3.2%) cats were bilateral inguinal and 5 (8.1%) cats were bilateral abdominal cryptorchids.
In conclusion, this represents the largest and most comprehensive study of cryptorchidism in cats and includes new data on pedigreed breeds. Cryptorchidism in general is less common in cats than in dogs. Pedigreed breeds of cats are more likely to be cryptorchid than random bred cats and the highest rate of cryptorchidism was found in the Ragdoll breed.
Cryptorchidism in the Cat
by Susan Little DVM, Dipl ABVP (Feline)
Introduction
Each testicle starts out inside the abdominal cavity of the kitten and descends into the scrotum after passing through the inguinal canal in the groin.
Normally, testicles are present in the scrotum at birth or shortly afterward, although they may be very small and difficult to detect.
By the age of 6 to 8 weeks, they should be large enough to be palpated. However, the testicles may not stay in the scrotum permanently until 4 to 6 months of age.
Until that time, the cremaster muscles can retract immature testicles into the groin.
Cryptorchidism refers to the failure of one or both testicles to descend into the scrotum and remain there by 7 to 8 months of age.
While unilateral cryptorchidism is most common, it can be bilateral. The mode of inheritance is suggested to be recessive and polygenic, and cryptorchid males should not be used for breeding.
Unilateral cryptorchid males may be fertile and so should be castrated.
Is It An Inherited Trait ?
Cryptorchidism is an example of a sex-limited trait.
The trait is physically expressed only in the male even though it can be carried by females.
Both the sire and dam of an affected cat should be considered to be carriers.
Some full siblings of an affected cat will also be carriers. A reduction in the number of cryptorchid cats in a breeding program can be achieved by removing affected males and carrier parents from breeding.
If the problem is widespread in a family line, full siblings of an affected cat should also be eliminated from the breeding program.
A young bilaterally cryptorchid male cat showing absence of testicles but spines present on the penis. The spines indicate that there is a source of testosterone present.
How Common Is It ?
In dogs, the incidence of cryptorchidism has been estimated at 1.2%.
In contrast, a study done at the Animal Medical Center, New York found that the rate of cryptorchidism in cats seen at that institution was 3.8%.
The most commonly represented purebred cat in this survey was the Persian (20% of cases).
Other represented breeds included the Himalayan (6%) and Siamese (4%). DSH cats represented 50% and DLH cats represented 6%. Most cryptorchids were unilateral (90%) and of these cats, the left and right sides were equally affected.
In cats where the location of the retained testicle was recorded in the medical record, 49% were in the groin, 33% were in the abdomen, and 14% were within the inguinal ring.
Two cats in this study (2%), a Burmese and an Abyssinian, were monorchid (only one testicle was present). Spermatic cords and vessels were present but ended blindly with no testicle.
Another study done at Michigan State University looked at the incidence of cryptorchidism in cats over a 10-year period (1980-1989). This study found an incidence of 1.7%. Two cats were monorchid (0.1%).
Persians were also over represented in this study with an incidence of 29%. Most cryptorchids were unilateral (78%) in this study as well. The bilateral cryptorchids all had retained testicles present in the abdomen. Tissues from abdominally retained testicles were examined and no sperm were found.
It is likely that abdominally retained testicles are sterile because the higher temperature inside the body will suppress development of sperm.
Cats with retained testicles located outside the abdomen, however, may be fertile.
The penis of a neutered male cat has no spines. They disappear within 6 weeks of castration.
© 2021 Berkano Cattery – All material on this site is copyrighthed and many not be printed or reproduced, in any form,without the express writen consent of Berkano Cattery.
Página desarrollada con ❤ por Cattery Web.

